RESEARCH
Agronomed will leverage its partnership with Drexel to facilitate world-class research. More specifically, some areas of focus will include:

Development of a patient registry study which will employ observational methods to collect uniform data to evaluate specified outcomes for a population defined by a particular disease, condition, or exposure. Value Creation Potential: Licensing Data, uncovering opportunities for product formulation and IP
In partnership with Drexel, which has the one of the largest comprehensive HIV care services in the country, Agronomed will explore PTSD and chronic neuropathic pain, which are the most common comorbidities in HIV patients. Pharmacological treatment for PTSD and neuropathic pain, especially in this patient population remains as an unmet medical need. Therefore, we plan to evaluate the clinical effectiveness of medical cannabis on PTSD, neuropathic pain and HIV through our patient registry and a well-designed clinical research program. Value Creation Potential: Licensing Data, uncovering opportunities for product formulation and IP
-
Agronomed to work closely with Drexel and associated hospitals to perform real time, pre-clinical and clinical trials
-
Establish patient registry with systems capturing a 360 view of the patient that is critical for performing anecdotal research including interactions, side effects and most effective dosing
-
Utilize research data and results to:
-
Publish findings to the state, medical practitioners, and NIH / NIDA advancing provider adoption through evidenced based medicine
-
Refine existing product efficacy and develop new products product through rapid patient quality feedback loops
-
Catalyze per patient, customized integrated medicine
-
-
Utilize Drexel IRB for review and execution of cost effective pre-clinical and clinical trials
-
Total investment over 5 year period is 8.4MM which is included in proforma
-
Leverage NIH and Federal University grants to accomplish more advanced trials
-

PARTNERSHIP OVERVIEW
PARTNERSHIP ADVANTAGES
-
Leverage University laboratory resources, expertise and methods to drive better:
-
Medicine
-
Provider Education
-
Patient Adoption
-
Patient Outcomes
-
Student Education
-
Industry Employee Prospects
-
-
Leverage academic resources and research to access NIDA, NIH, and other research grants
-
Target / apply for a DEA schedule 1 license (federal research permit)
-
Shared IP and patent development driving increased company and market valuation
-
Provides potential and experience to establish research incubator that could amass additional revenues, employment options and IP for the partnership

RESEARCH PRIORITIES
Agronomed would work collaboratively with Drexel / Hahnemann to:
-
Identify parallel interest areas
-
Identify availability of required expertise (or recruit as needed)
-
Perform market analysis of existing patents and research already underway
-
Develop management team, sponsorship, and working teams required to assess above inputs and develop a roadmap for which sub-project prioritization can be performed (IRB, RAC and/or others)
-
Sub-projects will be reviewed by boards for prioritization approval
Sub-projects / studies will fall into one or more of the following categories
-
Observational study: study design > IRB application > begin project
-
Pre-Clinical: Mostly In Vitro or In Vivo driven studies (controlled environment)
-
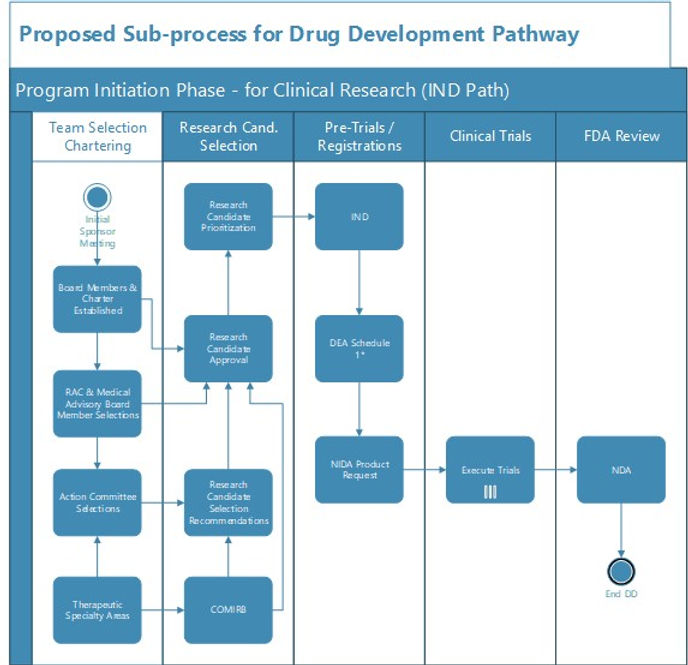
Clinical Trial: Study design --> IRB application --> file IND --> obtain DEA


RESEARCH PRIORITIES

CLINICAL TRIAL SUB PROCESS
OPPORTUNITIES CREATED
-
Bio-Pharma Incubator
-
Furthering of research
-
-
Building IP Value through
-
Patenting of new formulations
-
Licensing Trial Data
-
Big Pharma Partnerships or Product Sale
-
-
Direct Patient Sale
